
CAPA Webinar Summary
Identifying Non-conformances There are non conformances in your business right now, how…

Food Facility Checks To Do Before Your Food Safety Audit
Door Gap Checks Inspect your food facility doors (and your dock doors)…

Changes to BRCGS Global Standard Packaging Materials Issue 7 Audit Expectations
Follow these steps to prepare for your upcoming BRCGS Packaging Materials Issue…

Amazon Food Safety Expectations for Suppliers
The Amazon requirement for third-party food safety certification applies to: Chilled and…

Backflow Preventers in Food Industry Facilities
What is a Backflow Preventer and Where are They Needed? Backflow preventers…

Food Facility Restrooms: Requirements and Best Practices
What are the Restroom Requirements for GFSI Facilities? Several GFSI schemes (i.e….

Internal Audit Preparation Webinar – Recording and Summary
Internally Auditing to a Standard If you are desiring for your facility…

Top 12 Grocery Stores & Retailers Requiring Food Safety Audits for Suppliers
Albertsons Food Safety Audit Expectations According to Albertsons, all food and food-contact…

Whole Foods Market Suppliers Need Food Safety Certification
Six Steps to Prepare for Your Whole Foods Market Food Safety Certification…

Episode 6: Dietary Supplement Certification
Dietary Supplements Differ from Food As we have reviewed throughout this series,…

Episode 5: GMPs in a Dietary Supplement Program
Hygiene Programs While your facility may have one set of processes to…
Episode 4: Testing in Dietary Supplement Operations
What we are Testing for Because 21 CFR 111 does not require…

Episode 3: Best Practices for Dietary Supplement Operations
The Master Manufacturing Record The Master Manufacturing Record, or MMR is the…
Episode 2: Regulatory Framework of Dietary Supplements
21 CFR 111 Looking at 21 CFR part 111, there are 16…

Episode 1: Introduction to Dietary Supplement Manufacturing
Definition of a Dietary Supplement According to the FDA, “A dietary supplement…

Episode 1: Introducing Validation and Verification
Definition of Validation & Verification The concepts of validation vs. verification are…

Episode 3: Long Term Planning of Validation & Verification
Validation in our Daily Lives Looking at validation of the larger facets…

Episode 4: Short Term Planning for Validation and Verification
Short-term Validation Assessment Benefits Where the sources of long-term validation in a…

Ep 5: Validation & Verification of Critical Safety & Quality Equipment
Validating Equipment for a Recipe Let’s begin by looking at validation of…

Episode 6: Regulatory Requirements and Challenge Studies
FDA regulated facilities that produce other types of food other than juice…
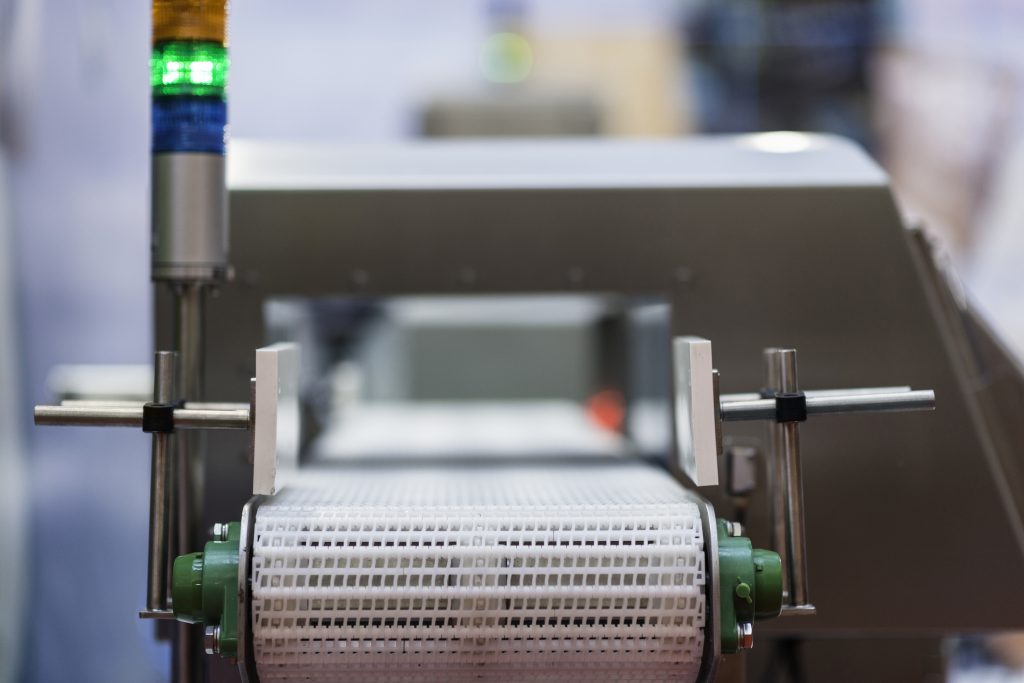
Episode 7: Validation and Verification of Metal Detectors and X-rays
Controversy About X-Rays and Metal Detectors Metal detectors and x-rays are commonly…

Ep 8: Validation & Verification in Quality & Safety Certification Programs
ISO quality management requirements and GFSI programs operate on many similar programs,…
Questions about costs, timelines and requirements?
Contact Us Today for a Free Consultation
Available to travel for your project
-
Headquarters

-
Offices


