Dietary Supplement Consulting
We stay with you until you pass. No matter what.
100%
Pass Rate
900+
Happy Clients
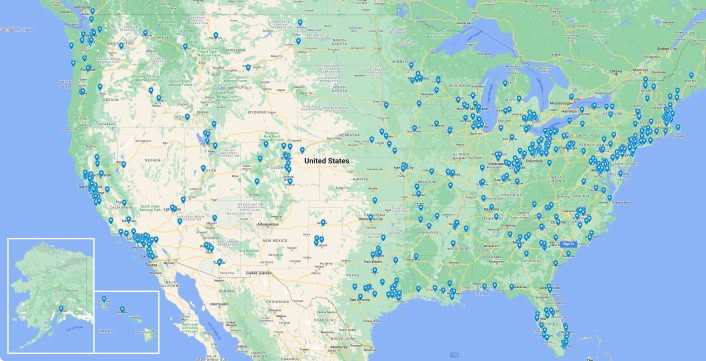
50
States
Get Your Dietary Supplement Certification Program Written, Implemented & Certified Quickly & Efficiently
We write & implement Dietary Supplement certification programs, including current good manufacturing practices (cGMP) & compliant with NSF/ANSI 455-2 & 21 CFR 111 (& accepted by Amazon & other major retailers).
Our clients’ dietary supplement certification success rate is 100% because we stay with our clients until you pass, no matter what.
Our dietary supplement consultants (bilingual English/Spanish available) are responsive, straightforward, and dedicated to our clients.
We Deliver Dietary Supplement Certification Programs at 4X Faster Turnaround and a 100% Audit Success Rate
How do we do it? By working closely with your team and by following our repeatable and reliable process.
Kellerman Consulting’s team of expert food safety & dietary supplement consultants knows what it takes for your products to get certified.
Our Dietary Supplement Consultants Write & Implement Programs in Compliance with FDA 21 CFR Part 111 and Deliver :
Consistent Results
All of our full-time consultants are well-versed in Kellerman Consulting’s repeatable and reliable process. Our team’s collaborative culture gives you the benefit of our collective experience with hundreds of successful outcomes. You will achieve the same performance & 100% success rate regardless of which of our team members you work with.
Quality Control and Accountability
You benefit from the support of consultants who review every project as a team. The co-founders of Kellerman Consulting are involved with each project at every stage to make sure that we are delivering on our commitment to our clients.
Responsiveness
Our full-time dietary supplement consultants respond immediately to all client questions, which reflects one of our core values. Kellerman Consulting team members are kept up to date on every project, so that any one of our consultants can easily step in at any moment to give you the reliability and responsiveness your project deserves.
Employee Food Safety Training Resources
In addition to your written Dietary Supplement certification program, every one of our clients receives access to a package of employee food safety training videos, quizzes, and training logs that are not publicly available at no additional cost in both English and Spanish.

-

We’ll assess your unique process and needs.
-

Our team will write your Dietary Supplement program customized to your operation.
-

We’ll train you and your team on how to follow all of the dietary supplement procedures.
-
Implement your program with us right beside you.
-

Pass your audit with support from our team.
DIETARY SUPPLEMENT Q & A:
What is a dietary supplement?
Dietary supplements are intended to be consumed in addition to your regular food diet. Common examples of dietary supplements are: probiotics, vitamins, minerals, herbs, and more. Nutraceuticals are in the same category as dietary supplements and food additives according to the FDA.
Who is responsible for reviewing dietary supplement products?
Dietary Supplements are treated as a food, and not as a drug by the FDA. Be cautious not to make label claims or health claims about treating or curing diseases, which would cause the product to be considered a drug instead of a dietary supplement. The FDA sometimes inspects dietary supplement manufacturing and processing facilities to assess regulatory compliance. In addition to FDA compliance, many dietary supplement companies choose to go through a voluntary third-party certification process. Kellerman Consulting will write your dietary supplement certification program, we’ll train you to implement it, and we’ll stay with you until you get certified.
What is 21 CFR Part 111?
21 CFR Part 111 is the section of the Code of Federal Regulations that covers Current Good Manufacturing Practice (cGMP), Packaging, Labeling & holding for Dietary Supplements. Regulatory requirements and FDA resources for dietary supplements may be found here. Kellerman Consulting’s food safety and dietary supplement consultants provide a written dietary supplement certification and regulatory compliance program that is specific to your product and your dietary supplement business, and we train you to follow your dietary supplement program to pass your next certification or inspection. We stay with you until you pass. No matter what.
What is Dietary Supplement and Vitamin Certification?
The Dietary supplement market is large and competitive, therefore, many companies choose to get third-party cGMP certification to help their products stand out. There are a variety of companies offering certification services. Kellerman Consulting is unaffiliated with any certifying body. NSF’s supplement certification is widely recognized in the supplement industry. NSF states, “use of NSF consulting services or attending NSF training sessions does not provide an advantage, nor is it linked in any way to the granting of certification”. Kellerman Consulting’s team of expert food safety & dietary supplement consultants knows what it takes for your products to get NSF certified.

Learn From Our Clients’ Experiences
Questions about costs, timelines and requirements?
Contact Us Today for a Free Consultation
Available to travel for your project
-
Headquarters

-
Offices