ISO 13485 Consulting for the Medical Device Industry
We stay with you until you pass. No matter what.
100%
Pass Rate
900+
Happy Clients
50
States
Get Your ISO 13485 Certification Program for Medical Devices Written, Implemented, and Certified Quickly
ISO 13485 is an international standard published by the International Organization for Standardization (ISO) for medical device quality management systems. The FDA published the Quality Management System Regulation (QMSR) final rule to align US medical device industry regulations more closely with ISO 13485. The FDA will begin to enforce the QMSR requirements, February 2, 2026.
Kellerman Consulting is an ISO 9001:2015 certified consulting company and we have assisted 900+ clients throughout the supply chain to get their programs written, implemented and certified.
Whether you are looking to get ISO 13485 certified, or if you need to comply with the QMSR final rule, we can offer the solution that is right for your business. Kellerman Consulting has full-time employees on staff who have experience writing and implementing ISO 13485 certification programs and have worked in the medical device industry. We stay with you until you pass. No matter what.
What to Expect During Your ISO 13485 Consultation
During your free ISO 13485 consultation, you’ll learn what the requirements are for getting certified, how much it costs with no hidden fees, how long it takes, and how our process works to assure you pass your ISO 13485 certification audit on the first try.
Our ISO 13485 Consulting Process Delivers a 4X Faster Turnaround and a 100% Success Rate
How do we do it? By working closely with your team and by following our repeatable and reliable ISO 13485 consulting process to help get your medical device business ISO 13485 certified. We will write and implement your ISO 13485 QMS and tailor policies and procedures to your satisfaction and to meet certification standards.
Our ISO 13485 Consultants Deliver
Consistent ISO 13485 Certification Results to Grow Your Core Medical Device Business
All of our ISO 13485 consultants are full-time employees and are well-versed in Kellerman Consulting’s repeatable and reliable ISO 13485 process for your medical device industry business. Our team’s collaborative culture gives you the benefit of our collective experience with hundreds of successful outcomes. You will achieve the same 100% success rate regardless of which of our team members you work with.
Quality Control and Accountability to Meet Certification Standards
You benefit from the support of ISO 13485 consultants who review every project as a team. Kellerman Consulting’s Senior Management team members are involved with each project at every stage to make sure that our consultancy is delivering on our commitment to our clients and ensuring customer satisfaction. Protect and develop your core business with support from our ISO 13485 consulting team.
Responsiveness
Our full-time ISO 13485 consultants respond immediately to all client questions, which reflects one of our core values. Kellerman Consulting team members are kept up to date on every project, so that any one of our consultants can easily step in at any moment to give you the reliability and responsiveness your project deserves.

-

We’ll assess your unique process and ISO needs.
-

Our team will write your ISO 13485 quality management standard program customized to your organization and your device.
-

We’ll train you and your team on how to follow all of the ISO quality management system procedures.
-
Implement your ISO program with our ISO consultant right beside you.
-

Pass your ISO audit with support from our team.
ISO 13485 Q&A:
What is ISO Certification?
ISO certification is a voluntary certification process to verify if a service, product, or system meets the standard requirements of the International Organization for Standardization (ISO).
What Types of Companies Get ISO 13485 Certified?
Companies who get ISO 13485 certified are part of the design, production, installation, storage, development, and servicing of medical devices. Businesses which manufacture medical devices or supply services to medical device manufacturers often choose to get ISO 13485 certified in order to ensure the quality of products and services. ISO 13485 certification is a well-recognized international certification standard that is specific to the medical device industry.
How is the Medical Device Industry Regulated in the United States?
The Food and Drug Administration (FDA) regulates the medical device industry in the United States. Medical device manufacturers must seek FDA approval by showing evidence that a device is safe and effective before a medical device can be sold in the United States.
What is the Difference Between ISO 13485 and FDA Part 820?
ISO 13485 is an international standard, and 21 CFR Part 820 is specific to the United States. 21 CFR Part 820 is mandatory in the United States and it is required for medical device distribution in the United States, whereas ISO 13485 is a voluntary certification scheme.
In January of 2024, the FDA published a final rule to align its Quality System Regulation (QSR) with ISO 13485. The updated FDA part 820, called the QMSR will be effective Feb. 2, 2026.
Is ISO 13485 Mandatory?
ISO 13485 certification is not mandatory, however, retailers and businesses in the medical device supply chain may require companies to be ISO 13485 certified prior to doing business together.
Learn From Our Clients’ Experiences
Questions about costs, timelines and requirements?
Contact Us Today for a Free Consultation
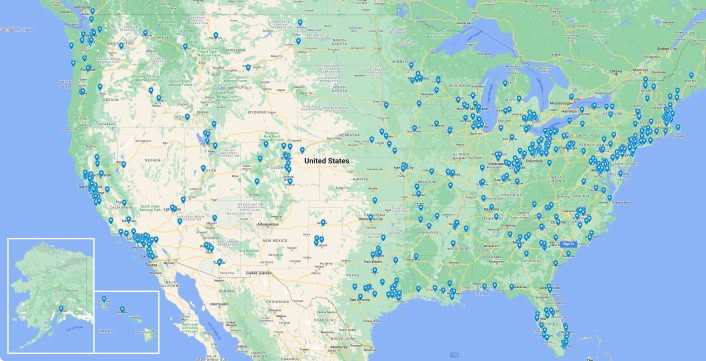
Available to travel for your project
-
Headquarters

-
Offices