Episode 8: Quality Issues In EMP programs
Episode 8: Quality Issues In EMP programs
Environmental Monitoring Programs
In episode eight of Kellerman Consulting’s Environmental Monitoring Program video series, we shift our attention to indicator testing and quality testing, which have different requirements than pathogen testing.
The most important distinction between pathogen testing and indicator or quality testing is that unlike pathogens, where we cannot have any detectable presence, we are allowed to have some presence of indicator or quality organisms.
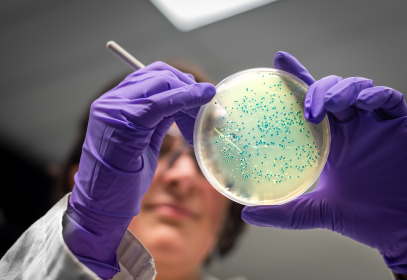
Since indicator organisms such as Aerobic bacteria and coliforms are allowable in some amounts of environmental testing, we will look at how to understand testing for APC, Coliforms, Yeast, Mold and other spoilage organisms, as well as how to set limits on results and determine what is acceptable and unacceptable.
The actions required for using swabs as part of an environmental monitoring program are the same when we test for indicator and quality testing, as we discussed in our previous episodes about pathogen swabbing. When indicator and quality testing organism swabs are prepared for zone testing, they should be scheduled, recorded and the tests submitted to a qualified lab.
One important difference between indicator and quality testing and pathogen testing is that with indicators and quality organisms, it is acceptable to swab food contact surfaces. But remember, we cannot swab food contact surfaces for pathogens unless instructed to do so by a regulator such as with an listeria control for ready to eat products in a meat and poultry facility. We can swab contact surfaces for indicators or quality organisms because they are not classified as posing an immediate safety risk for end users.
When trying to determine how to set limits for indicator organisms, the quality and safety personnel should review requirements from customers to see if limits are set externally. For facilities that do not have customer requirements, setting limits should ideally be done through analyzing results of repeated testing to get a baseline or average result.
In a previous episode we reviewed actions to take when a pathogen is discovered in the facility. These actions should be considered for indicator organisms where adverse results are discovered. At a minimum, the facility should clean and sanitize those areas where elevated levels are found. This should be followed by swabbing the affected spots after corrective action is performed to see if those actions were effective.
Lastly, we should be monitoring those areas more frequently following adverse results, and we should continue to target those areas for monitoring until we see trends that are consistently below the acceptable limits.
Thank you for watching. For free downloads to accompany this video series, visit the free training videos & resources page of our website.
Subscribe to our YouTube channel or follow us on LinkedIn to be notified of new educational food safety resources.